The mesomeric effect, also known as resonance effect or conjugation effect, is a fundamental concept in organic chemistry. It plays a crucial role in understanding the behavior and reactivity of organic compounds. This article aims to provide a comprehensive overview of the mesomeric effect, its implications, and its significance in the field of organic chemistry.
Table of content
- Introduction
- Understanding Resonance
- Mechanism of the Mesomeric Effect
- Types of Mesomeric Effects
- Application in Organic Compounds
- Influence on Chemical Reactivity
- Factors that can influence or override the mesomeric effect
- Experimental Techniques
- Conclusion
- FAQs (Frequently Asked Questions)
Now, let
us delve into each section in detail.
1. Introduction
The
mesomeric effect refers to the formation of polarity in the molecule by electron-donating
or electron-withdrawing substituents. It is formed due to the resonance
stabilization of delocalized electrons. It occurs when π (pi) bonds or lone
pairs of electrons are spread over multiple atoms through the overlap of
p-orbitals.
2. Understanding Resonance
Resonance
is a concept in chemistry that describes the delocalization of electrons within
a molecule or ion. It occurs when a molecule can be represented by multiple
resonance structures that differ in the placement of electrons while
maintaining the same overall connectivity of atoms.
3. Mechanism of the Mesomeric Effect
The
mesomeric effect arises from the overlap of p-orbitals, allowing electrons to
delocalize and move between atoms within a molecule. This delocalization of
electrons leads to stabilization or destabilization of the molecule, depending
on the nature of the substituents.
3.1 Comparison of Mesomeric and Inductive Effects
Mesomeric
effects and inductive effects are both electron-donating or
electron-withdrawing phenomena. However, they differ in their mechanisms and
the extent of electron distribution. Mesomeric effects involve the
delocalization of electrons through resonance, resulting in a broader electron
distribution. On the other hand, inductive effects operate through the
transmission of electron density along a sigma bond, leading to a localized
electron distribution.
4. Types of Mesomeric Effects
There are
two main types of mesomeric effects: positive mesomeric effect (+M), negative
mesomeric effect (-M). The positive mesomeric effect involves the donation of
electron density by a substituent, while the negative mesomeric effect
withdraws electron density.
4.1 Positive Mesomeric (+M) Effect
The +M
effect, also known as the positive mesomeric effect, involves the donation of
electron density by a functional group or atom to a neighboring atom or group.
This donation occurs through the overlap of π orbitals or through the
interaction of lone pairs of electrons. The +M effect enhances the electron
density at the receiving atom or group, stabilizing positive charges and
electron-deficient species. Examples of functional groups that exhibit the +M
effect include amino groups (-NH2) and hydroxyl groups (-OH).
| Fig 1: Positive Mesomeric (+M) Effect |
4.2 Negative Mesomeric (-M) Effect
In
contrast to the +M effect, the -M effect, or the negative mesomeric effect,
involves the withdrawal of electron density from a neighboring atom or group.
This withdrawal occurs when a functional group or atom possesses a higher
electronegativity or an electron-withdrawing capability. The -M effect reduces
the electron density at the receiving atom or group, stabilizing negative
charges and electron-rich species. Examples of functional groups that exhibit
the -M effect include carbonyl groups (C=O) and nitro groups (-NO2).
 |
| Fig 2: Negative Mesomeric (-M) Effect |
5.
Application in Organic Compounds
The
mesomeric effect plays a significant role in various organic compounds. In
aromatic compounds, it influences the stability and reactivity of the aromatic
system. In carbonyl compounds, it affects the polarity and reactivity of the
carbonyl group. Additionally, in conjugated systems, the mesomeric effect
determines the electronic and optical properties.
6.
Influence on Chemical Reactivity
The
mesomeric effect has a profound impact on the chemical reactivity of organic
compounds. It stabilizes reaction intermediates such as carbocations, carbanions, and
free radicals, thereby influencing reaction rates and product formation. It
also affects the acidity and basicity of compounds, as well as the nucleophilic
and electrophilic behavior.
6.1
Stabilization of intermediates
The
stabilization of intermediates in chemical reactions through the
mesomeric effect is a crucial aspect of organic chemistry. Intermediates are
transient species that form during chemical reactions and play a vital role in
determining the reaction mechanism and product formation.
When an
intermediate is formed, it often possesses an unpaired electron or an
electron-deficient center. The mesomeric effect occurs when adjacent atoms or
groups in the molecule have π (pi) bonds or lone pairs of electrons that can
donate or withdraw electron density through resonance. This delocalization of
electrons can stabilize the intermediate, making it less reactive and more
likely to persist in the reaction pathway.
The
positive mesomeric effect (+M) involves the donation of electron density to the
intermediate, while the negative mesomeric effect (-M) withdraws electron
density from the intermediate. The electron-donating groups, such as alkylgroups (-R), amino groups (-NH2), or hydroxyl groups (-OH), can stabilize
positive charges or radicals in the intermediate by sharing their electron
density through resonance. On the other hand, electron-withdrawing groups, such
as carbonyl groups (-C=O) or nitro groups (-NO2), can stabilize negative
charges in the intermediate by withdrawing electron density through resonance.
By
stabilizing intermediates, the mesomeric effect can affect the reaction rate
and selectivity. Stabilized intermediates are less likely to undergo undesired
side reactions or decomposition and are more likely to participate in
subsequent steps of the reaction. This stabilization can also influence the
overall yield of the desired product.
For
example, consider the formation of a carbocation intermediate during a
substitution reaction of 3-bromobut-1-ene in MeOH/ H2O. The adjacent alkyl
group can donate its electron density through resonance, stabilizing the
positive charge on the carbocation and making it less reactive. This
stabilization increases the lifetime of the carbocation, allowing it to react
with the nucleophile and form the desired substitution product.
 |
| Fig 3: Stability of Carbocation by Mesomeric effect |
Conversely,
carbanions are stabilized by negative mesomeric effect. For example, in the
reaction of diethyl malonate with methyl bromide under basic conditions. Here
the intermediate carbanion is stabilized by negative mesomeric effect of
carbonyl groups.
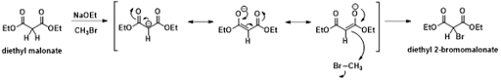 |
| Fig 4: Stability of Carbanion by Mesomeric effect |
Similarly,
in radical reactions, the mesomeric effect can stabilize the radical
intermediate, preventing it from undergoing undesired side reactions or
termination processes. This stabilization enhances the efficiency of radical
chain reactions and controls the selectivity of radical reactions.
In
conclusion, the mesomeric effect plays a crucial role in stabilizing
intermediates during organic reactions. By donating or withdrawing electron density
through resonance, adjacent atoms or groups can influence the stability and
reactivity of these intermediates. Understanding and harnessing the mesomeric
effect can provide chemists with valuable tools for designing and controlling
chemical reactions.
6.2
Impact on acidity and basicity
The
mesomeric effect, also known as resonance effect or conjugation effect, has a
significant impact on the acidity and basicity of organic compounds. Acidity
refers to the tendency of a compound to donate a proton (H+) or release a
positively charged species, while basicity refers to the tendency to accept a
proton or donate a pair of electrons. The mesomeric effect can influence the
distribution of electron density within a molecule, affecting its acidity or
basicity.
The
mesomeric effect can either enhance or decrease the acidity or basicity of a
compound, depending on the nature of the substituents and the electron-donating
or electron-withdrawing nature of the groups involved.
Acidic compounds:
For example,
acidity of carboxylic acid such as para substituted benzoic acid is affected by
mesomeric effect. Electron donating groups like methoxy group (OCH3) increase
the electron density in the carbonyl functional group. Therefore, electron
donating groups at para position of benzoic acid decreases the acidity.
Conversely,
electron withdrawing groups like nitro group (NO2) withdraw electron density
from carbonyl functional group. Therefore, electron withdrawing groups at para
position of benzoic acid increases the acidity.
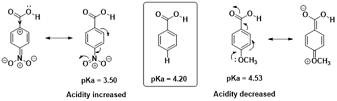 |
| Fig 5 : Impact of Mesomeric Effect on Acidity of Compound |
Basic compounds:
Positive
mesomeric effect (+M): Electron-donating groups can increase the basicity of a
compound by stabilizing the positive charge or donating electron density to the
electron-pair donor. A classic example is aniline (-NH2), where the lone pair
of electrons on the nitrogen atom can be delocalized into the benzene ring,
enhancing its basicity.
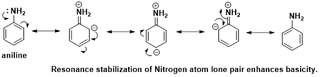 |
| Fig 6: Impact of Mesomeric Effect on Basicity of Compound |
Negative
mesomeric effect (-M): Electron-withdrawing groups can decrease the basicity of
a compound by withdrawing electron density from the electron-pair donor. For
instance, amide are weaker base than corresponding amine. This is because in
case of amide, the carbonyl group (-C=O) withdraw electron density from the
adjacent amino group (-NH2), reducing its ability to donate electrons and
decreasing basicity.
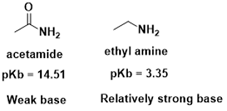 |
| Fig 7 : Electron withdrawing group can decrease the Basicity of compound |
It is
important to note that the mesomeric effect is just one of several factors that
contribute to the acidity and basicity of a compound. Other factors, such as inductive
effects and solvation effects, can also influence the overall acidity or
basicity.
In
conclusion, the mesomeric effect can significantly impact the acidity and
basicity of organic compounds. The electron-donating or electron-withdrawing
nature of substituents can either enhance or decrease the acidity or basicity,
depending on the specific molecular context. Understanding the mesomeric effect
is essential for predicting and explaining the behavior of organic compounds in
acid-base reactions.
6.3
Effects on nucleophilic and electrophilic reactions
The
mesomeric effect, also known as the resonance effect or conjugation effect,
plays a crucial role in nucleophilic and electrophilic reactions in organic
chemistry. It can significantly influence the reactivity and outcome of these
reactions by affecting the distribution of electron density within a molecule.
Nucleophilic reactions:
Positive
mesomeric effect (+M): Electron-donating groups can increase the
nucleophilicity of a compound by donating electron density to the atom or group
that acts as the nucleophile. This donation of electron density enhances the
availability of electrons for nucleophilic attack. For example, in the reaction
of a nucleophile with a carbonyl compound, the presence of electron-donating
groups (such as alkyl groups) adjacent to the nucleophilic center increases the
electron density on the carbon atom, making it more susceptible to nucleophilic
attack.
For
example, Grignard reagent like, ethyl magnesium bromide is strong
nucleophile due to mesomeric effect of ethyl group.
 |
| Fig 8 : Alkyl Grignard Reagent is a Strong Nucleophile due to Mesomeric Effect |
Negative
mesomeric effect (-M): Electron-withdrawing groups can decrease the
nucleophilicity of a compound by withdrawing electron density from the atom or
group acting as the nucleophile. This withdrawal of electron density reduces
the availability of electrons for nucleophilic attack. For instance, the
presence of electron-withdrawing groups (such as nitro groups) adjacent to a
nucleophilic center decreases its nucleophilic reactivity.
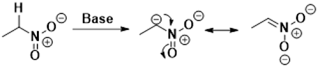 |
| Fig 9 : Electron withdrawing group can decrease Nucleophilicity |
Electrophilic
reactions:
Positive
mesomeric effect (+M): Electron-donating groups can enhance the
electrophilicity of a compound by donating electron density to the atom or
group that acts as the electrophile. This donation of electron density
increases the positive charge or electron deficiency on the electrophilic
center, making it more attractive to nucleophiles. For example, in electrophilic
aromatic substitution reactions, the presence of electron-donating groups (such
as alkyl groups) on the aromatic ring increases the electron density on the
carbon atom, making it more reactive towards electrophiles.
Negative
mesomeric effect (-M): Electron-withdrawing groups can decrease the
electrophilicity of a compound by withdrawing electron density from the atom or
group acting as the electrophile. This withdrawal of electron density reduces
the positive charge or electron deficiency on the electrophilic center, making
it less attractive to nucleophiles. For instance, the presence of
electron-withdrawing groups (such as carbonyl groups) on a carbocation
decreases its electrophilic reactivity.
It is
important to note that the mesomeric effect is just one of several factors that
influence nucleophilic and electrophilic reactivity. Other factors, such as
steric hindrance, solvent effects, and the nature of the nucleophile or
electrophile, also play significant roles in determining the outcome of these
reactions.
In
conclusion, the mesomeric effect has a profound impact on nucleophilic and
electrophilic reactions in organic chemistry. The electron-donating or
electron-withdrawing nature of substituents can enhance or decrease the
reactivity of a compound, affecting its nucleophilicity or electrophilicity.
Understanding and manipulating the mesomeric effect is essential for
controlling and designing organic reactions.
7.
Experimental Techniques
Several
experimental techniques are utilized to study and understand the mesomeric
effect, a fundamental concept in organic chemistry. These techniques provide
insights into the distribution of electron density within molecules, allowing
researchers to analyze the impact of the mesomeric effect on various properties
and reactivities. Some commonly employed experimental techniques include:
UV-Vis Spectroscopy: UV-Vis spectroscopy involves the absorption of ultraviolet and
visible light by molecules. This technique can provide information about the
electronic transitions occurring in a compound, allowing researchers to assess
the extent of electron delocalization caused by the mesomeric effect. By
analyzing the absorption spectra, the presence and magnitude of the mesomeric
effect can be determined.
Nuclear
Magnetic Resonance (NMR) Spectroscopy: NMR spectroscopy is a powerful technique
used to analyze the structure and behavior of molecules in solution. Through
NMR experiments, researchers can investigate the electronic environment and
chemical shifts of atoms within a molecule. By comparing the chemical shifts of
atoms involved in mesomeric effects with reference compounds, valuable insights
can be obtained regarding electron delocalization and the mesomeric effect.
X-ray Crystallography:
X-ray crystallography is a technique that provides detailed information about
the three-dimensional structure of a molecule. By analyzing the positions of
atoms and the electron density distribution, researchers can gain insights into
the extent of electron delocalization caused by the mesomeric effect. X-ray
crystallography allows for visualizing and confirming the presence of resonance
structures and their impact on the overall structure of a molecule.
Electron
Paramagnetic Resonance (EPR) Spectroscopy: EPR spectroscopy is employed to
study compounds with unpaired electrons or radicals. By analyzing the EPR
spectra, researchers can gain information about the electron distribution and
spin states of molecules. This technique can provide valuable data on the
extent of electron delocalization and the mesomeric effect in paramagnetic
species.
Computational
Methods: Computational methods, such as density functional theory (DFT)
calculations, are widely utilized to study and quantify the mesomeric effect.
These methods involve solving mathematical equations that describe the behavior
of electrons within molecules. By performing calculations, researchers can
predict and visualize the electron density distribution, resonance structures,
and energy levels associated with the mesomeric effect.
Combining
these experimental techniques with theoretical calculations allows researchers
to gain a comprehensive understanding of the mesomeric effect and its impact on
various chemical properties and reactions. The combination of experimental data
and computational modeling provides valuable insights into the electron
delocalization and resonance phenomena occurring within organic compounds.
8.
Factors that can influence or override the mesomeric effect
While the
mesomeric effect is a powerful concept in organic chemistry, there are several
factors that can influence or override its effects. It is important to consider
these factors to understand the full picture of a molecule's reactivity and
behavior. Some key factors include:
Steric
Hindrance: Steric hindrance occurs when bulky substituents or groups restrict
the movement or interaction of electrons. If a substituent hinders the
delocalization of electrons through the mesomeric effect, it can weaken or
override the mesomeric effect. Steric hindrance can disrupt the alignment of
p-orbitals or hinder the overlap required for resonance, reducing the impact of
the mesomeric effect.
Conjugation
Length: The length of a conjugated system can also influence the mesomeric effect.
Longer conjugated systems generally exhibit stronger mesomeric effects because
they allow for greater electron delocalization. As conjugation extends over
more atoms, the electron density becomes more evenly distributed, enhancing the
mesomeric effect. Shortening the conjugated system or introducing
non-conjugated regions can weaken or even eliminate the mesomeric effect.
Electronic
Effects of Neighboring Functional Groups: The presence of neighboring
functional groups can influence the mesomeric effect. Electron-withdrawing or
electron-donating groups near the mesomeric system can alter the distribution
of electron density and affect the strength of the mesomeric effect. These
neighboring groups can either reinforce or counteract the mesomeric effect,
depending on their nature and position.
Solvent
Effects: The choice of solvent can also impact the mesomeric effect. Solvents
can stabilize or destabilize charged species through solvation effects, thereby
altering the electron distribution and the strength of the mesomeric effect.
Polar solvents tend to enhance the mesomeric effect, while nonpolar solvents
may weaken or disrupt it.
Electronic
Effects of Functional Groups: The presence of other functional groups within a
molecule can influence the mesomeric effect. Electron-withdrawing or
electron-donating functional groups can compete with the mesomeric effect,
altering the distribution of electron density and affecting the overall
electronic properties. The combined influence of various functional groups
needs to be considered when evaluating the mesomeric effect.
Understanding
these factors and their interplay is crucial for predicting and interpreting
the behavior of molecules in organic chemistry. While the mesomeric effect is a
significant factor, it is important to consider the broader electronic and
structural context to fully comprehend the reactivity and properties of a
compound.
9.
Conclusion
In
conclusion, the mesomeric effect is a vital concept in organic chemistry that
explains the behavior and reactivity of organic compounds. It provides insights
into the distribution of electron density within molecules, influencing their
stability, reactivity, and physical properties. Understanding the mesomeric
effect is crucial for designing and predicting the behavior of new compounds in
the field of organic chemistry.
10. FAQs
(Frequently Asked Questions)
Q1: How
does the mesomeric effect differ from the inductive effect?
The
mesomeric effect involves the delocalization of electrons through resonance,
whereas the inductive effect is the electron-donating or electron-withdrawing
effect of substituents through sigma (σ) bonds.
Q2: Can
the mesomeric effect occur in non-aromatic compounds?
Yes, the
mesomeric effect can occur in non-aromatic compounds that contain conjugated
systems or multiple bonds.
Q3: What
is the significance of the mesomeric effect in drug design?
The
mesomeric effect helps in understanding the electronic and steric properties of
drug molecules, aiding in the design of more potent and selective drugs.
Q4: How
can the mesomeric effect be quantified experimentally?
The
mesomeric effect can be studied using various spectroscopic techniques, such as
UV-Vis spectroscopy and NMR, and computational methods like DFT calculations.
Q5: Are
there any exceptions to the mesomeric effect?
Yes,
there are exceptions to the mesomeric effect, which can be influenced by
factors like steric hindrance, conjugation length, and neighboring functional
groups.
That is all for this topic, keep exploring and uncovering the
wonders of chemistry! see you in the next blog. Thank you.
No comments:
Post a Comment