Hi Friends, in this article we will see how to calculate coupling constants (J values) in 1H-NMR Spectroscopy.
Key words: Coupling constant, Multiplicity, 1H-NMR spectrum, Signal.
Definition of coupling constant
The coupling constant is a distance between sub-peaks expressed in hertz. In 1H-NMR spectrum a peak splits into multiple sub-peaks due to coupling with neighboring protons. The value of coupling constant does not depend upon frequency of machine and solvent used for NMR experiment.
We have discussed various applications of spectroscopy in chemistry, medicine and environmental science in another article. Please check out for more details. [Link]
Introduction
In proton
nuclear magnetic resonance (NMR) spectroscopy, the coupling constant is a key parameter that
provides valuable information about the molecular structure and bonding in a
compound. The coupling constant, denoted as J, represents the splitting of NMR
signals observed in a spectrum due to the magnetic interactions between
neighboring hydrogen atoms (protons) in a molecule. In this article, we will
explore the importance and implications of coupling constants in proton NMR.
Understanding Coupling Constants
The
magnitude of the coupling constant is measured in hertz (Hz) and indicates the
strength of the magnetic interaction between the coupled protons. It is
determined by the nature and distance of the chemical bonds between the protons
involved.
The
coupling constant provides information about the number of neighboring protons
and their relative arrangement with respect to the proton of interest. By
analyzing the pattern and splitting of the NMR peaks, chemists can deduce the
connectivity and structural features of the molecule under investigation.
Typically,
the coupling constant is described by two parameters: the coupling constant
value (J) and the coupling multiplicity. The coupling constant value reflects
the size of the splitting, while the coupling multiplicity describes the number
of peaks observed in the NMR spectrum.
For
example, in a simple case of two coupled protons, known as a
"doublet," the coupling constant reflects the strength of the
interaction between the two protons. The doublet appears as a pair of peaks,
usually of equal intensity, with a splitting pattern determined by the coupling
constant.
Factors Affecting Coupling Constants
The value of the coupling constant depends on several factors, including
- Bond length between the coupled protons,
- Nature of the bonding (single, double, or triple bond), and
- Hybridization state of the carbon atoms attached to the protons.
Different types of bonds and molecular environments can give rise to
distinct coupling constants.
Applications of Coupling Constants
The
coupling constant is useful in structural elucidation and can provide
information about the connectivity, stereochemistry, and conformational
properties of organic molecules. It serves as a powerful tool for chemists to
analyze and interpret proton NMR spectra, allowing them to determine the
structure and understand the behavior of organic compounds.
Interpretation of Coupling Constants
Multiplicity
of signals and calculation of respective J values is discussed below.
Singlet
A
singlet peak is represents single line. Hence there will not be any coupling
constant for this peak.
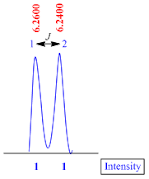
Doublet
A doublet is consisting of two sub peaks with 1:1 intensity.
The
coupling constant (J value) for doublet peak is calculated by the formula;
J
value = (line 1 – line 2) x frequency of NMR machine
Suppose
the NMR recorded in 400 MHz machine, therefore
J
value = (6.2600 – 6.2400) x 400
J
value = 0.02 x 400
J
value = 8.0 Hz
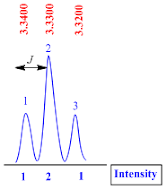
Triplet
A
triplet peak is consisting of three sub peaks and they have 1:2:1 intensity.
The
coupling constant (J value) for triplet peak is calculated by the formula;
J
value = (line 1 – line 2) x frequency of NMR machine
Suppose
the NMR recorded in 400 MHz machine, therefore
J
value = (3.3400 – 3.3300) x 400
J
value = 0.01 x 400
J
value = 4.0 Hz
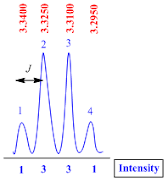
Quartet
A quartet peak is consisting of four sub peaks with 1:3:3:1 intensity.
The
coupling constant (J value) for quartet peak is calculated by the formula;
J
value = (line 1 – line 2) x frequency of NMR machine
Suppose
the NMR recorded in 400 MHz machine, therefore
J
value = (3.3400 – 3.3250) x 400
J
value = 0.015 x 400
J
value = 6.0 Hz
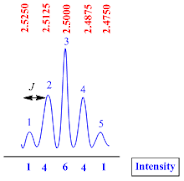
Quintet
A quintet
peak is consisting of five sub peaks. They have 1:4:6:4:1 intensity.
The
coupling constant (J value) for quintet peak is calculated by the formula;
J
value = (line 1 – line 2) x frequency of NMR machine
Suppose
the NMR recorded in 400 MHz machine, therefore
J
value = (2.5250– 2.5125) x 400
J
value = 0.0125 x 400
J
value = 5.0 Hz
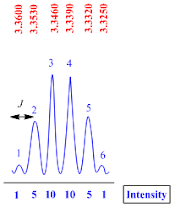
Sextet
A
sextet peak is consisting of six sub peaks. They have 1:5:10:10:5:1 intensity.
The
coupling constant (J value) for sextet peak is calculated by the formula;
J
value = (line 1 – line 2) x frequency of NMR machine
Suppose
the NMR recorded in 400 MHz machine, therefore
J
value = (3.3600– 3.3530) x 400
J
value = 0.007 x 400
J
value = 2.8 Hz
In second order spectra the splitting pattern is more complex and it may have more than one coupling constant.
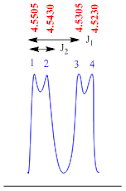
Doublet of doublets
A doublet of doublets peak is consisting of four lines. This is second order splitting pattern and it appears as two doublet peaks.
The
coupling constant (J1 value) for doublet of doublets peak is
calculated by the formula;
J1
value = (line 1 – line 3) x frequency of NMR machine
Suppose
the NMR recorded in 400 MHz machine, therefore
J1
value = (4.5505– 4.5305) x 400
J1
value = 0.02 x 400
J1
value = 8.0 Hz
The
coupling constant (J2 value) for doublet of doublet peak is
calculated by the formula;
J2
value = (line 1 – line 2) x frequency of NMR machine
J2
value = (4.5505– 4.5430) x 400
J2
value = 0.0075 x 400
J2
value = 3.0 Hz
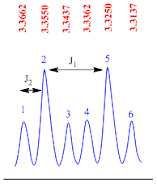
Doublet of triplets
A doublet of triplets peak is consisting of six sub peaks. This is second order splitting pattern and it appears as two triplets.
There
are two coupling constants found in this type of splitting pattern. The
coupling constant (J1 value) for doublet of triplets peak is calculated by the
formula;
J1
value = (line 2 – line 5) x frequency of NMR machine
Suppose
the NMR recorded in 400 MHz machine, therefore
J1
value = (3.3550– 3.3250) x 400
J1
value = 0.03 x 400
J1
value = 12.0 Hz
The
coupling constant (J2 value) for doublet of doublet peak is calculated by the
formula;
J2
value = (line 1 – line 2) x frequency of NMR machine
J2
value = (3.3662– 3.3550) x 400
J2
value = 0.0112 x 400
J2
value = 4.5 Hz
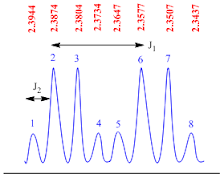
Doublet of quartet
A
doublet of quartets peak is consisting of eight sub peaks. This is second order
splitting pattern and it appears as two quartets.
There
are two coupling constants found in this type of splitting pattern. The
coupling constant (J1 value) for doublet of triplets peak is calculated by the
formula;
J1
value = (line 2 – line 6) x frequency of NMR machine
Suppose
the NMR recorded in 400 MHz machine, therefore
J1
value = (2.3874– 2.3577) x 400
J1
value = 0.0297 x 400
J1
value = 11.8 Hz
The
coupling constant (J2 value) for doublet of doublet peak is calculated by the
formula;
J2
value = (line 1 – line 2) x frequency of NMR machine
J2
value = (2.3944– 2.3874) x 400
J2
value =0.007 x 400
J2
value = 2.8 Hz
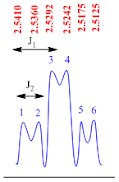
Triplet of doublets
A
Triplet of doublets peak is consisting of eight sub peaks. It appears as three
doublet peaks. This is second order splitting pattern.
There
are two coupling constants found in this type of splitting pattern. The
coupling constant (J1 value) for triplet of triplets peak is calculated by the
formula;
J1
value = (line 1 – line 3) x frequency of NMR machine
Suppose
the NMR recorded in 400 MHz machine, therefore
J1
value = (2.5410– 2.5292) x 400
J1
value = 0.0118 x 400
J1
value = 4.7 Hz
The
coupling constant (J2 value) for triplet of triplets peak is calculated by the
formula;
J2
value = (line 1 – line 2) x frequency of NMR machine
J2
value = (2.5410– 2.5360) x 400
J2
value = 0.005 x 400
J2
value = 2.0 Hz
That's all for this topic. If you have any questions please feel free to ask me in the comment box.
Also, we have discussed splitting and multiplicity pattern in another article. please see the link below;
Topics in Organic Chemistry: Splitting and Multiplicity in Proton NMR (chemistrywithdrsantosh.com)
Thank you..!


Nice mitra
ReplyDeleteThank you.
ReplyDeleteWelcome... Your message means a lot to me.
ReplyDeleteThank you friend it is well written
ReplyDeleteWelcome... Please check out other articles as well.
Deleteits really valuable information ......
ReplyDeleteThank you.
DeleteThank you for simplified this topic with excellent information
ReplyDeleteWelcome..! Thank you very much for noticing the work.
DeleteSimply explain, very informative
ReplyDeleteTerima kasih, informasi berharga ini, mohon ada penjelasan lebih lanjut penerapan konstanta kopling dan hubungan dengan panjang ikatan, sifat ikatan dan keadaan hibridisasi atom C. Terima kasih
ReplyDeleteWelcome.
ReplyDeleteThank you very much. The information and explanation were a blessing. God bless and replenish you abundantly.
ReplyDeleteWelcome. Thank you for noticing the work.
Delete