Key words: Purification, Organic compounds, Chromatography, Crystallization.
Table of Contents
- Introduction
- Methods of purification of organic compounds
- Conclusion
1. Introduction
There are important methods available for purification of organic compounds. These are;
1. Sublimation
2. Precipitation
3. Trituration
4. Crystallization
5. Distillation
6. Column chromatography
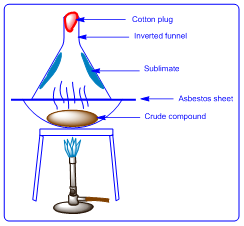
1.1. Sublimation
Certain
chemical compounds such as Ammonium chloride, Carbon dioxide, Camphor
Napthalene etc has tendency to undergo phase transition directly from solid to
gas phase. This property of substances is called as sublimation. Hence these kinds
of substances are purified by heating the compound to form vapours, and then
the vapours are collected in inverted funnel. After cooling the vapours pure
substance deposits on the walls of funnel. The impurities that do not have
sublimation property would remain in the heating vessel.
In this method of purification the solid compound is dissolved in suitable solvent to form clear solution. Then to the obtained solution; another solvent is added in which the given solid compound is not soluble. Hence the compound settles at the bottom as precipitate. Finally the mixture is filtered to remove soluble impurities and pure compound is obtained.
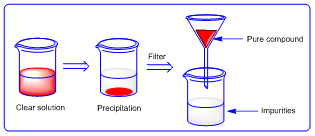 |
| Figure 2: Precipitation |
1.3. Trituration
This is method of purification used for purification of organic compounds to remove insoluble impurities. In the method impure compound is heated in suitable solvent to dissolve compound. The impurities would be insoluble and the solution is filtered in hot condition to get clear solution. Finally the solvent is evaporated to pure compound.
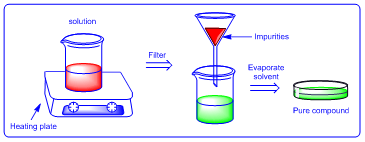 |
| Figure 3: Trituration |
1.4. Crystallization
This is frequently used method for purification of organic as well as inorganic compounds. In this method given compound is mixed in solvent and resulting solution is heated. Then it allowed cooling up to room temperature to form crystals. Finally crystals are separated by filtration.
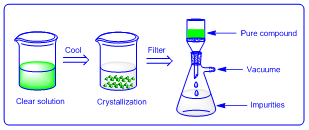 |
| Figure 4: Crystallization |
1.5. Distillation
It is a method which is used for purification of liquid compounds. The liquid compounds boils at specific temperature, this is known as boiling point of that compound. In the process of distillation; the liquid compounds are heated at boiling point temperature so that vapours formed. At this temperature impurities may not vaporise. Then pure vapours are cooled by using condenser (an apparatus which is filled with cold water). After cooling the vapours forms droplets and they are collected in clean flask.
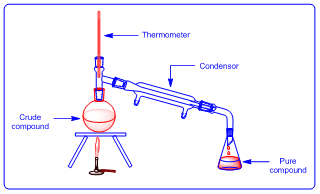 |
| Figure 5: Distillation |
1.6. Column chromatography
It is a method which is used for liquid and solid compounds. In this method a cylindrical glass burette is used which is known as “column”. The column is filled with silica. Then compound which is to be purified is mixed with small amount of silica and added on the top. Finally solvent is added preferably mixture of solvents. Then solvent flows through the column in downward direction. Each compound has tendency to bind with silica in weaker or stronger extent. The weakly bounded compound flows faster with solvent. And strongly bounded compound flows slower with the solvent. Then various fractions of solvents are collected which has similar compound. Finally all the collected fractions are concentrated to get pure compounds.
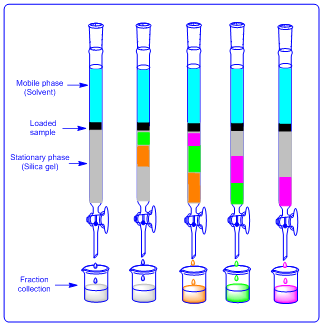 |
| Figure 6: Column chromatography |
No comments:
Post a Comment