UV-Vis spectroscopy is a branch of spectroscopy is deals with absorption of light in ultraviolet and visible region.
Hi Friends, in this article we will learn about basics of UV visible spectroscopy and also see its applications in chemistry. Here we will discuss transition metal complexes and Beer-Lambert Law.
Keywords: UV-Vis spectroscopy, Coordination Complex, Beer-Lambert Law, P bond.
We have discussed various applications of spectroscopy in chemistry, medicine and environmental science in another article. Please check out for more details. [Link]
Table of Contents
- Introduction
- Absorption of Light
- What is P bond system
- Transition metal complexes
- Why we see colours?
- How does UV-Visible Spectrum look like?
- What is Beer-Lambert Law?
- Applications of UV Spectroscopy
- Conclusion
1. Introduction
UV-Vis spectroscopy is a branch of spectroscopy is deals with absorption of light in ultraviolet and visible region. The atoms and molecules absorb light and undergo electronic transitions. The absorption spectroscopy refers to measurement of transitions from the ground state to the excited state.
Various
spectral regions of electromagnetic spectrum (light) are listed below;
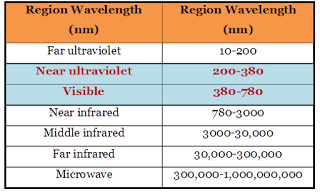 |
| Figure1: Regions of electromagnetic spectrum |
2. Absorption of Light
The
uv-spectroscopy deals with absorptions of light in the range of 200-800 nm. The electrons of atoms or molecules
absorb precise light in the ultraviolet and visible region to go from one level
to another energy level.
The possible electronic transitions that light might cause are given below. In each of the case; an electron from filled orbital excited to go in empty high energy orbital.
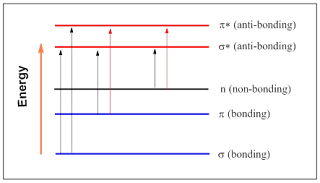 |
| Figure 2: Electronic transitions |
Every
transition requires precise amount energy. The larger the gap between the
energy levels, the greater the energy required to promote the electron to the
higher energy level. The ultraviolet and visible lights can cause only two
transitions; that is
- p-bonding
to p*-(anti-bonding)
- non-bonding
to p*-(lone
pair)
Therefore to absorb light between the range 200-800 nm, the molecular must consists of p bond or any atom with lone pair (oxygen, nitrogen or halogen).
3. What is p bond system?
A p bond is formed due to side overlap of half-filled p orbitals. For example; ethene molecule is having one p bond which is formed by the overlap of p orbitals of two carbon atoms. In the ground state both the electron found in p –bonding energy level. These electrons absorb light and excites to p* –anti bonding energy level.
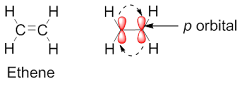 |
| Figure 3: Orbital diagram of ethene |
Some
examples of p-bond systems given below;
 |
| Figure 4: p-bond systems |
If the
energy gap between π bonding orbitals and π* anti-bonding orbitals is more; the
molecule absorbs light of higher energy and shorter wavelength. Similarly, if
the energy gap between π bonding orbitals and π* anti-bonding orbitals is less;
the molecule absorbs light of lower energy and longer wavelength.
In
conjugated p-systems as the amount of delocalization increases; the
energy gap between the π bonding orbitals and π* anti-bonding orbitals gets
smaller. Therefore the molecule absorbs light of lower energy and longer
wavelength.
4. Transition metal complexes
The
transition metal complexes tend to absorb UV and visible light. This is due to
splitting of d orbital of central metal ion. When the legand forms bond with
metal ion, in this process some of the d orbitals gets energy and some lose energy.
Hence the d orbital gets divide by energy. The amount of splitting or energy
gap is depending upon ligand and central metal ion.
For
example; Hexaaquacobolt(II) ion complex [CO(H2O)6].
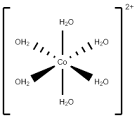 |
| Figure 5: Hexaaquacobolt(II) ion complex |
In this case five 3d orbitals of cobalt ion divide in to 3 low energy orbitals and 2 high energy orbitals. The electrons from low energy orbital absorb light and excites to higher energy orbital.
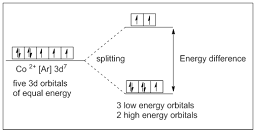 |
| Figure 6: Representation of orbital structure of Hexaaquacobolt(II) ion complex |
5. Why we see colour?
When
visible light fall on a substance, then characteristic portion of light get absorbed. And remaining portion of light is reflected.
The reflected light is appears as colour of that substance. It is also known as
complementary colour of absorbed wavelength of light. This relationship is can
be understood by colour wheel. Here it is seen that complementary colours are diametrically
opposite each other. That means if a substance absorbs “red” light then it will appears as “green”
colour.
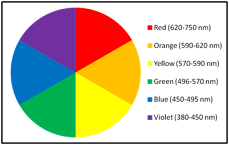 |
| Figure 7: The color wheel |
6. How does UV-Visible Spectrum look like?
The UV-Visible spectrum is a graph which is plotted between absorbance (vertical axis) and wavelength (horizontal axis). The wavelength which corresponds to maximum absorption is referred as “lambda max” (l max). Each substance or compound has specific l max value.
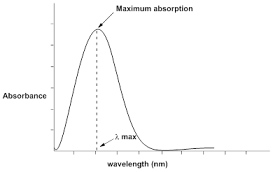 |
| Figure 8: Typical UV -spectrum |
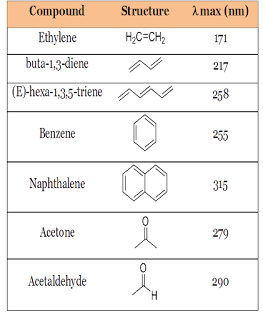
Maximum
absorbance values of some organic compounds as given below;
7. What is Beer-Lambert Law?
The Beer-Lambert
Law states that “absorbance is directly proportional to the concentration of
the compound in solution”.
According to Beer-Lambert
Law the UV-visible spectroscopy can also be
used to measure the concentration of a sample. If we know the absorption value
(A) and molar extinction constant (e) of a compound then we can calculate
the concentration of unknown solution.
8. Applications of UV Spectroscopy
To measure concentration of unknown solution:
A plot of absorbance verses
concentration of series of samples is linear if they follow Beer-Lambert Law.
This graph is known as Calibration graph.
And based on absorbance value of the unknown sample it is easy to find
concentration of respective solution.
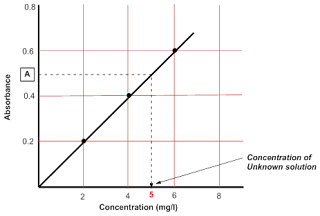 |
| Figure 10: The Calibration graph |
To
study Reaction kinetics:
Concentration of reactant or product changes
in the course of reaction. Hence absorbance also changes as the reaction
progress. Then a plot of absorbance verses time can give the idea about order
of the reaction with respect to the reactant or product. This also helps to
study the reaction mechanism.
To determine Dissociation constants (pKa) of acids and bases:
It
is possible to determine dissociation constant of compound if the acid / base
form of the compound absorbs UV light.
pKa of compound can be calculated by
using Henderson–Hasselbalch equation
pH = pKa + log [A-] / [HA]
The ratio [A-] / [HA] can be
calculated by plotting the graph between absorbance versus wavelength at
respective pH value.
Detection of Impurities:
The UV spectrum of sample is compared with that of standard
reference. Here Additional peaks can be observed due to impurities. Hence we
can identify amount of impurities in the sample.
Structure elucidation of organic compounds:
Cis-trans isomers , presence or absence of unsaturation can
be identified by using UV-spectroscopy.
9. Conclusion
To summarize this article, we have
learned basics of UV-spectroscopy and its applications. Here electronic state
transitions due to ultra violate and visible light are responsible for
absorption of light. If the compound absorbs light in the visible region we see
its complementary color. UV-spectroscopy can be utilized by measurement of
concentration of compound in solutions, and structure elucidations of organic
compounds.
That's all for this topic. Thank you..!

No comments:
Post a Comment