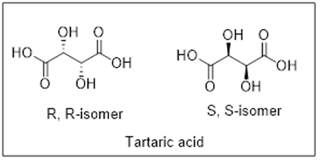
As we
know that a chiral compound consists of two types of isomers or enantiomers. If a sample consist of only one isomer, then such sample is
called as optically pure. But if the given sample consists of both the isomers
in same or different proportions, then the purity of compound is specified by
percent optical purity.
Definition of Optical purity
Optical
purity of compound is defined as percentage of major isomer present in the
sample of compound.
Formula
for percent optical purity is;
 |
| Figure : Percent optical purity |
So,
to calculate the percent optical purity we need to add specific rotation values
of sample and pure enantiomer in above equation.
Let’s
see this with one example;
A sample of tartaric acid shows specific
rotation of +8 so what will be its percent optical purity?
To solve this problem, first we need to
know optical rotation value of pure isomers.
The R,R-isomer of tartaric acid has specific rotation of -12 ° whereas S,S-isomer has specific rotation of +12°. Since the sample has positive specific rotation value, it means that the sample consists of S,S-isomer in major proportion.
Therefore, according to the formula;
Hence percent optical purity of given
sample is 66.6 %. In other words, the sample consists of S,S-isomer of tartaric
acid in 66.6 %.
So, amount of R,R-isomer in the sample =
100-66.6 = 33.4 %
Now we will learn about enantiomeric
excess.
Definition of Enantiomeric excess
The enantiomeric excess value shows
which isomer is present in greater extent.
Enantiomeric excess is calculated by
the formula;
Hence the enantiomeric excess of the
given sample is 33.2 %
Now take one more example to calculate
percent optical purity and enantiomeric excess.
Suppose a stereoselective reduction of
2-butanone provides chiral 2-butanol. The isolated compound shows specific
rotation -10.5. So, what will be its optical purity and enantiomeric excess?
 |
| Figure : Stereoselctive reduction of 2-butanone |
Here
first thing we need to find specific rotation of pure R-isomer and S-isomer. It
is reported that (R)-2-butanol has specific rotation of +13.9 ° whereas
(S)-2-butanol has specific rotation of −13.9 °.
Since the
specific rotation value is negative that means the sample has major S-isomer. Therefore;
Hence
it is understood that the isolated compound has S-isomer with 75.5 %
The
percentage of R-isomer = 100−75.5 = 24.5 %
Now
we will calculate Enantiomeric excess
Therefore,
isolated compound from stereoselective reduction has 75.5 % optical purity and
51 % enantiomeric excess.
I hope that this article would been
informative for you. Please write in the comment section below if you have any
queries regarding this topic. Also, you can suggest me subjects for next
articles.
Thank you. See you in next blog.
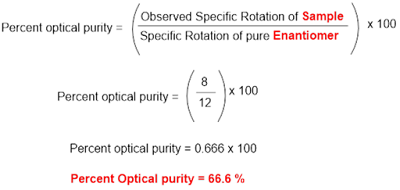



No comments:
Post a Comment